광배근 근피판을 통한 두피 재건 및 두개골성형
Scalp Reconstruction and Cranioplasty using the Latissimus Dorsi Musculocutaneous Flap in a Patient with Recurrent Wound Dehiscence Accompanied by MRSA Infection
Article information
Abstract
= Abstract =
The latissimus dorsi flap has high vascularity and is helpful for the reconstruction of infected areas. Herein, we present a patient with recurrent infections and soft-tissue defects who underwent cranial reconstruction using a free latissimus dorsi flap. The patient had undergone craniectomy and reconstruction using alloplastic bone 18 years previously. A scalp defect accompanied by infection occurred five years ago, and patient underwent reconstruction using a free flap at another hospital; however, the problem persisted. After debridement and bone flap removal, the right latissimus dorsi musculocutaneous flap was elevated, and the thoracodorsal artery and vein were anastomosed end-to-end to the right superficial temporal artery and vein. Methicillin-resistant Staphylococcus aureus was eradicated, and the flap survived. Cranioplasty was performed eight months later, and one year follow-up proceeded without complications. Effective reconstruction and cranioplasty are possible using the free latissimus dorsi musculocutaneous flap, even on scalp with persistent infections and soft-tissue defects.
Introduction
Infections after cranioplasty occur frequently,1) and slow wound healing due to insufficient blood supply makes the scalp vulnerable to infection.2) In cases of scalp defects due to tumor resection or trauma, free flap reconstruction is performed when resolving soft-tissue defects with local flaps is challenging. Moreover, free flaps with sufficient blood flow are resistant to bacteria.3) The latissimus dorsi (LD) flap has high vascularity and is helpful for the reconstruction of infected areas.4)
Herein, we present a case where the patient had undergone craniectomy and cranioplasty, but a subsequent infection aggravated the soft-tissue defect. Although reconstruction using a free superficial circumflex iliac artery perforator (SCIP) flap had already been performed to replenish the soft tissue, Methicillin-resistant Staphylococcus aureus (MRSA) infection persisted, and the soft-tissue defect was aggravated through repeated debridement. To address this issue, the infected lesion was completely excised and reconstructed using a free LD flap with high vascularity.
Case report
A 45-year-old man was injured in a motorcycle accident 18 years ago and had undergone craniectomy and delayed reconstruction using alloplastic bone in the same year. Five years ago, a scalp defect accompanied by infection occurred, and he underwent reconstruction using a free SCIP flap at another hospital. However, the infection and soft-tissue defects persisted; therefore, he visited another hospital. Wound dehiscence continued to occur, even after three local flap surgeries; hence, the affected area was washed and negative pressure wound therapy was applied for one month, though the wound did not improve.
The patient presented at our hospital with a prolonged soft-tissue defect and scalp infection. On examination, there was a 25 × 25 mm soft-tissue defect in the right parietotemporal region, and the previously inserted alloplastic bone was exposed (Fig. 1). The evidence of infection was not prominent, and there was no pus or discharge. On computed tomography, there was no focal lesion in the brain parenchyma or CSF space other than cerebromalacia in the bifrontal lobe. It was challenging to resolve the soft-tissue defect with a local flap because the patient had previously undergone several debridement resections and three local flap surgeries. Therefore, free flap surgery had to be considered. Since infection could not be completely ruled out, a decision was made to remove the exposed bone flap. In addition, extensive debridement including the previously reconstructed SCIP flap—to remove the source of infection as much as possible—was performed. Afterward, reconstruction using a free LD musculocutaneous flap with high vascularity was planned.
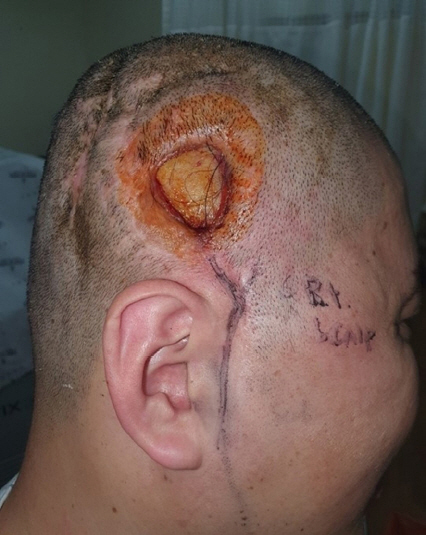
Preoperative soft-tissue defect in the right parietotemporal region. A soft-tissue defect measuring 25 × 25 mm is seen on the scalp, and the alloplastic bone used for the previous cranioplasty is exposed under it. CT showed that cranioplasty was performed in the right parietotemporal area, and there was no focal lesion in the brain parenchyma or CSF space other than cerebromalacia in the bifrontal lobe. CT, computed tomography; CSF, cerebrospinal fluid.
The area around the soft tissue defect was debrided, and the scalp was resected (Fig. 2A). The bone flap was removed with the artificial dura, and the dural defect was repaired (Fig. 2B). Culture tests were requested for the alloplastic bone, SCIP flap tissue, artificial dura swab, and wound swab. Subsequently, the right LD musculocutaneous flap was elevated with a 16 × 9 cm skin paddle and a muscle dimension of 15 × 15 cm, and the thoracodorsal artery and vein were anastomosed end-to-end to the right superficial temporal artery and vein (Fig. 2C). Every culture test identified MRSA; therefore, IV vancomycin was administered. After the 25th day of IV antibiotics, the culture test showed negative MRSA, wound healing was completed, and the patient was discharged. Progress was continuously observed through outpatient procedures; since LD flaps were well engrafted and no wound problems, including infection, occurred, the patient was treated with titanium mesh cranioplasty eight months later. Although temporary flap congestion occurred immediately after surgery, it recovered without any problems due to intravenous PGE1 injection and application of NG cream for one week. The patient was followed-up without complications for one year (Figs. 3 and 4).
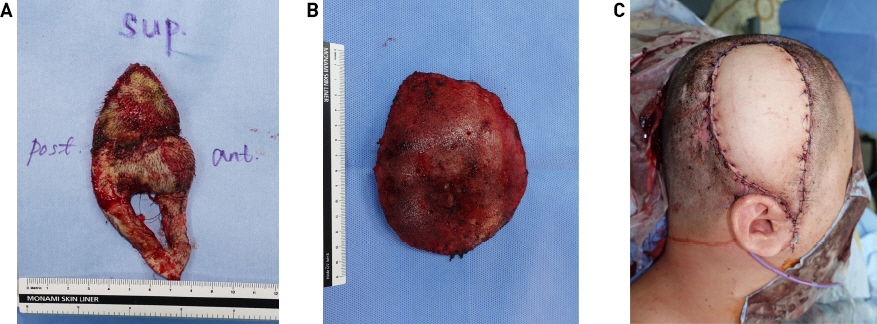
Medical photos immediately after surgery. (A) The previously used SCIP flap was excised around the soft-tissue defect. (B) The exposed bone flap was removed. (C) The LD flap was elevated with a 16 x 9 cm skin paddle with a muscle dimension of 15 × 15 cm and reconstructed by inset at the soft-tissue defect. SCIP, superficial circumflex iliac artery perforator (SCIP); LD, latissimus dorsi.
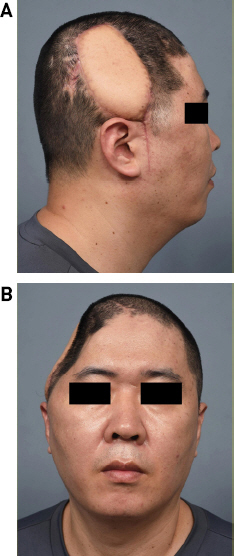
Medical photos after six months of reconstruction using free LD flap. The free LD flap was successfully engrafted without any complications. There were no signs of infection after surgery. (A) Lateral view. (B) Frontal view. LD, latissimus dorsi.
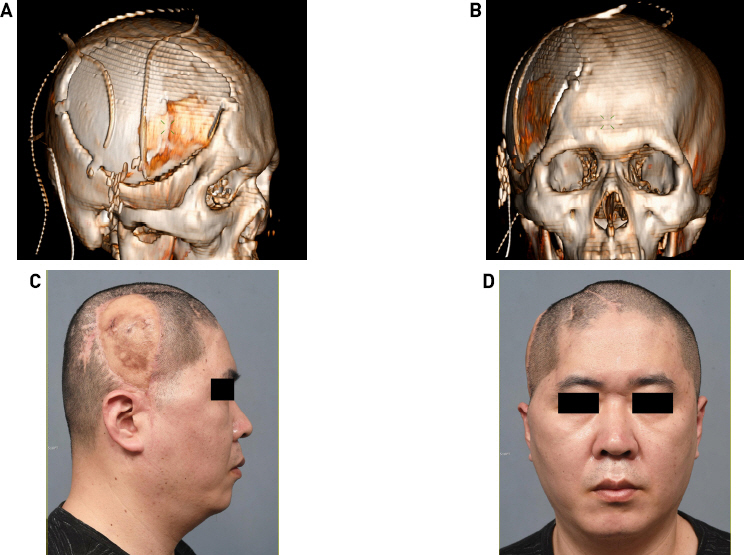
Computed tomography 3-D reconstruction image immediately after cranioplasty and medical photos nine months after surgery (17 months after free flap surgery). After eight months of reconstruction, the flap was stabilized, and cranioplasty was performed using titanium mesh. Postoperative CT 3-D reconstruction image. (A) Lateral view. (B) Frontal view. Flap congestion occurred immediately after surgery but recovered without any subsequent problems. This caused pigmentation to occur. (C) Lateral view. (D) Frontal view.
Discussion
Infections after cranioplasty is a common clinical problem, reported in 0% to 21.4% of cases, with an average of 7.9%.5) Uncontrolled infection can cause secondary soft tissue defects in the scalp, worsening the infection. In this case report, the patient’s soft-tissue defect due to cranioplasty was accompanied by infection that was unresolved for several years. Minor scalp defects can be treated using secondary healing or local flaps; however, free flap is used to treat major scalp defects.6) In this case, reconstruction using a free flap was selected because there was not enough soft tissue for reconstruction after repeated local flap surgery, and followed by cranioplasty under infection control and flap stabilization. Thorough removal of the infectious agent is critical to promote flap engraftment and reduce infection after cranioplasty. In our patient, the scalp defect and subsequent infection had spread to the alloplastic bone. Artificial dura and alloplastic bone were removed, and the entire SCIP flap previously used for reconstruction was resected.
Various free flaps are used for scalp reconstruction, depending on the circumstances and the operator’s preference. For scalp reconstruction, the size and location of the defect, the presence of residual periosteum, the condition of surrounding tissues, the presence of infection, and accompanying comorbidities should be considered.6-8) Free flap is an excellent treatment option for complex wounds accompanied by infection, calvarial defects, and dead spaces.7-9) In addition, when cranioplasty must be performed together as in this patient, reconstruction using a free flap is more helpful.9-11) In reconstruction using a free flap, the recipient vessels are determined in consideration of the scalp defect location and the surrounding tissue, and the required length of the free flap pedicle is also determined accordingly.
Despite the many advantages of reconstruction using a free flap, it is necessary to select an appropriate flap because the morbidity of the donor site is indispensable. The gracilis flap has advantages such as constant anatomy, plasticity, possibility of neurosensory flap, wide skin paddle, and ease of flap thickness control, and disadvantages such as additional skin grafting and difficult flap monitoring after surgery.12) The rectus abdominis flap advantages include long and thick pedicle, abundant vascular supply, flexible skin paddle design, adjustable flap thickness, and moderate donor morbidity; however, it may be too bulky for use in scalp reconstruction depending on the patient.13) The vastus lateralis flap has advantages such as ease of securing a pedicle of sufficient thickness, adjustable flap thickness depending on the presence or absence of muscle, and low donor morbidity; however, it has large variations in perforators’ position and course.14) The LD flap provides a particularly large flap and has a high vasculature, which is advantageous for scalp reconstruction.4,15) When using the LD flap, the morbidity of the donor site is relatively low; however, during head and neck surgery, the donor and recipient sites are adjacent, so it may be difficult for two teams to operate simultaneously.15) These flaps used for head and neck reconstruction commonly provide a long pedicle, a wide flap and generous soft-tissue, and have rich blood flow; therefore, the operator’s preference also plays an important role in flap selection.
To be resistant to infection, the vascularity of the flap used for reconstruction must be high. While the SCIP flap has the advantage of being thin, it lacks vascularity. In contrast, the LD flap is resistant to infection because of its sufficient vascularity,15) making it better suited for reconstructing infected sites or areas of high risk of infection than other free flaps.4) Culture test was performed in the operating room, and we identified MRSA in both the alloplastic bone and artificial dura, indicating that the patient had been exposed to chronic MRSA infection. IV vancomycin was used immediately after flap operation; no bacteria were identified three weeks later. This result demonstrates the resistance of the LD flap to infection, including MRSA. Moreover, the unresolved infection under the SCIP flap was controlled after switching to the LD flap in the same patient, suggesting that LD flaps can be useful in secondary reconstruction.
There is controversy around whether the one-stage or the two-stage surgery is superior when performing cranioplasty, including free flap surgery.11) For cranial defects accompanied by soft-tissue defects, when cranioplasty is performed in one stage, there is room for complications due to incomplete soft-tissue healing.11) Provided that infection is not already present, it is generally accepted that there is no difference in the infection rate between the one-stage and two-stage surgeries. Considering the culture test results in this case, the risk of infection following one-stage surgery was high due to addition of foreign bodies for reconstruction without complete eradication. If infection cannot be excluded entirely, performing reconstruction in two stages is safer. In patients with persistent infection, as in this case, more successful reconstruction can be expected if the second cranioplasty is performed after the soft tissue is completely healed following the first operation.
Effective reconstruction and cranioplasty were successfully performed using a free LD musculocutaneous flap on a scalp with soft-tissue defects and persistent MRSA infection. The use of free LD flaps with high vascularity may be an effective alternative for secondary reconstruction in MRSA- infected patients.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H- 2110-023-1260) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient provided written informed consent for the publication and the use of his images.
Author contribution
Conceptualization: TY, SK. Data curation: TY. Methodology: TY, SK. Visualization: TY. Writing - original draft: TY. Writing - review & editing: TY, SK.