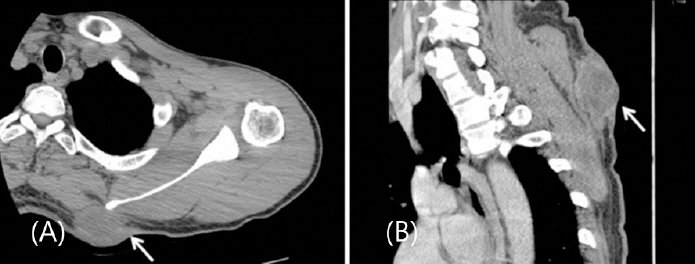
Fibrosarcoma usually affects very deep structures and tends to originate from the intramuscular and intermuscular fibrous tissue, myofascial integument, aponeurosis, tendons, with subfascial involvement.
4,
6,
7) Fibrosarcoma primarily occurs in areas composed of collagen-rich connective tissue; adult-type fibrosarcoma frequently develops in the lower extremities, particularly in the thighs and knees, along with the arms and trunk.
6) In contrast, the diagnoses of retroperitoneal, mediastinal, and head and neck fibrosarcomas are rare.
6) With the exception of dermatofibrosarcoma protuberans, fibrosarcoma arising from subcutis is rare and typically occurs in tissues damaged by radiation, heat, or scarring.
7) Several tests including immunohistochemical or microstructural analysis, must be performed in addition to routine histopathological examination for an accurate diagnosis of fibrosarcoma.
5) Certain mesenchymal tumors, including monophasic fibrous synovial sarcoma, leiomyosarcoma, malignant fibrous histiocytoma, malignant peripheral nerve sheath tumors, and other spindle cell tumors, have characteristics similar to those of fibrosarcoma.
5) Therefore, elucidation of the typical features of this tumor subtype is crucial for accurate diagnosis.
6) The exclusion of other spindle cell mesenchymal tumors is a vital step in diagnosis of fibrosarcoma in adults. Immunohistochemistry staining of adult fibrosarcoma is negative for CD34, CD99, bcl-2 and nuclear β-catenin, as well as epithelial, muscle, and neuronal markers.
2) Desmin, alpha smooth muscle actin (α- SMA), and muscle-specific actin (MSA) are among the most common myogenic markers.
6) Ki-67, a cell cycle-associated nuclear antigen, is also used as a prognostic marker for fibrosarcoma.
6) With respect to phenotype, the cell populations were S100 and CD68 negative, excluding schwannoma and malignant fibrous histiocytoma, respectively, whereas definitely positive only for vimentin.
2) Microscopic findings are important, in addition to immunohistochemistry. Adult fibrosarcoma is characterized by fusiform tumor cells with interlaced bundle formation and a herringbone pattern. Histopathological analysis in this case showed typical microscopic features of adult fibrosarcoma, with tumor cells arranged in long polycysts displaying a herringbone pattern. Immunohistochemical staining showed that the specimen was negative for CD34, S100, Desmin, SMA, and CD68, and showed a high Ki67 marker index (70%). The present case was representative of adult fibrosarcoma due to a combination of immunohistochemistry and microscopic features. The tumor did not exhibit significant skin layer changes, precluding the possibility of a fibrosarcoma area of dermal fibrosarcoma protuberans.
A wide resection must be performed if the tumor originates from the muscle cells or grows outside the compartment.
8) A margin of 2 cm is often recommended; however, a valid safety margin has generally not been established.
6) Further, radiation therapy after extensive resection is highly recommended for high-grade tumors up to 5 cm in size.
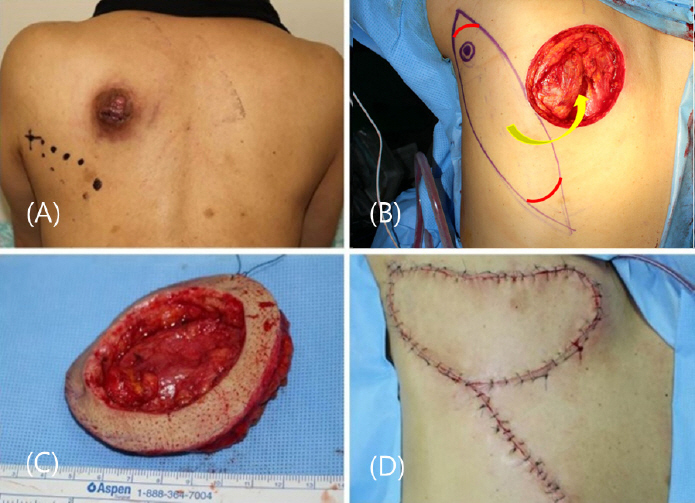
8) In this case, the tumor size was 5 cm with no muscle invasion. However, the base of the tumor was located adjacent to the muscle layer in the frozen biopsy obtained during the operation, and the malignancy was located higher compared to the dermatofibrosarcoma protuberance; therefore, a bowl- shaped resection was additionally performed. Consequently, a wide excision was performed, with a margin of 3.5 cm.
The defect site after tumor resection was reconstructed with a parascapular flap, which was chosen due to the absence of muscle defect in the recipient. The donor site that is adjacent to the lesion, and is a fasciocutaneous flap with reliable circulation. Barwick et al. (1982) reported that the five criteria for reconstruction of the defect using flaps: 1. applicability in a wide range of situations, 2. lengthy vascular pedicle of consistently large external diameter, 3. easy dissection, 4. minimal donor-site morbidity, and 5. uniformity of thickness and large surface dimensions.
9) The parascapular flap includes the skin and can be harvested at a maximum size of 30 cm × 15 cm, with a possibility of primary suturing of the donor site. Other advantages include an intradermal network with rich anastomosis and the constant anatomy of the vessels is well-known.
10) In addition, the morbidity of the donor site after surgery is low; however, a few motion limitations that are crucial for patients.
11,
12) During the follow-up period of three years after surgery, the patient did not experience any motional limitations, showed a symmetric back contour, and returned to daily work without functional limitations despite performing high-labor work. The patient was in very good overall condition and was working normally in delivery service. He underwent tumor removal and chemotherapy at a tertiary comprehensive hospital due to the presence of metastases in the lungs and pleura, and was under observation. Their last follow-up observation in the outpatient clinic was 3 years and 2 months ago.
To conclude, a concise histopathological diagnosis of each tumor is essential to establish a treatment plan for fibrosarcoma. This should be comprehensively determined using routine histopathological and immunohistochemical analyses. Adult-type fibrosarcoma of the scapular area is a rare case.
13) The overall five-year survival rate is approximately 40-60%.
6) The best prognosis should be achieved through an accurate diagnosis by the clinician, complete surgical resection of the tumor, and appropriate reconstruction. Therefore, despite a follow-up period of less than five years and metastases, there was no local recurrence, and the quality of life showed improvement. We expect that the data presented in our report may aid clinicians in handling similar challenging cases of adult FS.