Paclitaxel, Lenvatinib 및 방사선 병용 요법의 역형성 갑상선암에서의 항암 작용
Anti-cancer Activity of Paclitaxel, Lenvatinib and Radiation Combination Therapy on Anaplastic Thyroid Cancer in Vitro and in Vivo
Article information
Trans Abstract
Background/Objectives
Although anaplastic thyroid carcinoma (ATC) is rare, it is one of the deadliest forms of thyroid cancer. The fatality rate for ATC is high, and the survival rate at one year after diagnosis is <20%. The present study aimed to investigate the anti-tumor activities of paclitaxel, radiation, and tyrosine kinase inhibitor (TKI) combined therapy in anaplastic thyroid cancer cells both in vitro and in vivo and explore its effects on apoptotic cell death pathways.
Materials & Methods
ATC cell line was exposed to TKI, lenvatinib in the presence or absence of paclitaxel with radiation, and cell viability was determined by MTT assay. Effects of the combined treatment on cell cycle and intracellular signaling pathways were assessed by flow cytometry and western blot analysis. The ATC cell line xenograft model was used to examine the anti-tumor activity in vivo.
Results
Our data revealed that the combined administration of paclitaxel, TKI, and radiation decreased cell viability in ATC cells, and also significantly increased apoptotic cell death in these cells, as demonstrated by the cleavage of caspase-3 and DNA fragmentation. This combination therapy reduced anti-apoptotic factor levels in ATC cells, while significantly decreasing tumor volume and increasing survival in ATC xenografts.
Conclusion
These results indicate that administering the combination of paclitaxel, TKI, and radiation therapy may exert significant anticancer effects in preclinical models, potentially suggesting a new clinical approach for treating patients with ATC.
Introduction
Thyroid cancer is the most common endocrine malignancy comprising more than 90% of all endocrine cancers, and its incidence has increased in the last three decades [1]. Thyroid cancer is distinguished into well-differentiated, poorly differentiated, and anaplastic thyroid cancer according to the differentiation of cells and their ability to maintain the typical features of follicular cells [2]. Anaplastic thyroid cancer (ATC) is a rare, but an extremely aggressive malignancy, with a worldwide annual incidence of 1 to 2 cases per million [3]. Although it accounts only for 1% to 2% of thyroid carcinoma, it is responsible for more than 50% of all thyroid cancer-related deaths [4]. A significant event in the tumorigenic transformation is the constitutive activation of the RAS-BRAF-ERK signaling pathway, which is triggered by RET/PTC rearrangement [5]. BRAF and TP53 mutations have been reported to be the most prevalent mutations along with other frame-shift deletions and novel anaplastic gene fusions [6]. Mutations in genes involved in the PI-3K pathway are also commonly observed in ATC, supporting the development of advanced disease, as tumors acquire additional mutations [7]. ATC is characterized by lower expression levels of drug targets such as FGFRs, VEGFRs, KIT, and RET compared to both papillary thyroid cancers and normal tissues, explaining the observed resistance to therapies targeting these pathways [6].
The American Thyroid Association recommends surgery with locoregional radiation therapy with or without systemic therapy in resectable disease, and radiotherapy with or without systemic therapy in unresectable disease. The addition of chemotherapy to radiation therapy may potentially treat micrometastatic disease and affect long-term survival; however, it may also increase morbidity and mortality. Chemotherapy may be administered both as a radiosensitizing and an adjuvant approach. Chemotherapy agents recommended as therapeutics or used as a palliative approach include doxorubicin, cisplatin, and taxanes [8].
High-dose radiation therapy with total thyroidectomy has been shown to result in improved survival [9]. Chemotherapy followed by radiotherapy has been associated with a longer median survival, suggesting that a multimodal approach comprising surgery, radiotherapy, and chemotherapy needs to be adopted depending on the patient’s performance status [10]. Paclitaxel is the first member of the taxane family to be used as a chemotherapeutic agent. The taxanes exert a cytotoxic effect by arresting cells at mitosis through microtubule stabilization, which subsequently results in cellular apoptosis. The use of paclitaxel has become a broadly accepted option for treating solid tumors such as ovarian, breast, and non-small cell lung cancers [11]. Furthermore, paclitaxel is known to function as an effective induction or adjuvant therapeutic in ATC, helping to achieve long-term survival [12].
Lenvatinib was the second tyrosine kinase inhibitor ()to be tested for thyroid cancer in a phase III trial [13]. Lenvatinib is an oral, multi-targeted tyrosine kinase inhibitor of VEGFRs 1-3, FGFRs 1-4, PDGFR α, RET, and KIT signaling [14].
Regrettably, the majority of the patients do not respond to chemoradiation therapy and TKI, or respond at the beginning, but later develop resistance leading to tumor progression [15-18]. Several studies have been performed to overcome these problems by combining various chemotherapeutic drugs such as histone deacetylase inhibitors with paclitaxel, histone deacetylase inhibitors with sorafenib, and sorafenib with paclitaxel [19-21]. Since most patients with ATC are diagnosed at an advanced, inoperable stage, there is a need to develop novel clinical approaches for ATC treatment. The aim of this study was to determine the antitumor activities of the combination of paclitaxel, lenvatinib, and radiation therapy in ATC both in vitro and in vivo.
Materials and Methods
Patients and Tissue Specimens
Fresh tumors were obtained from patients with biochemically and histologically diagnosed ATC who were treated at the Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. Fresh tumors were acquired during surgical resection of the primary sites of thyroid cancer. The research protocol was approved by the Institutional Review Board of the Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine (IRB Protocol: 3-2019-0281, Cell samples were acquired from patients at the Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea).
Tumor Cell Isolation and Primary Culture
After resection, tumors were stored in normal saline with antifungal drugs and antibiotics and transferred to the laboratory. Normal tissues and fat were removed and the tissues were rinsed with 1× Hank's Balanced Salt Solution. Tumors were minced in a tube with dissociation medium containing DMEM/F12 with 20% fetal bovine serum supplemented with 1 mg/ml collagenase type IV (Sigma, St. Louis, MO, USA; C5138). Minced tissue and suspended tumor cells were filtered through sterile nylon cell strainers with 70-micron pores (BD Falcon, Franklin Lakes, NJ, USA), rinsed with 50 ml of 1× Hank's Balanced Salt Solution, and centrifuged at 220 g for 5 minutes. Cells were resuspended in RPMI-1640 (Hyclone, South Logan, UT, USA) medium with 10% fetal bovine serum (Hyclone) and 2% penicillin/streptomycin solution (Gibco, Grand Island, NY, USA). Cell viability was determined using the trypan blue dye exclusion method.
Cell Culture
The patient-derived ATC cells were isolated and grown in RPMI-1640 medium with 10% FBS (cells were authenticated by short tandem repeat profiling, karyotyping, and isoenzyme analysis). We decided call the patient derived ATC cells as KSA1.
Cell Viability Assay
Cell proliferation was measured using the MTT assay. To this end, cells were seeded in 96-well plates at 6×103 cells/well and incubated overnight to achieve 80% confluency. The indicated drugs were added at 0-100 μM final concentrations. Cells were incubated for the indicated times prior to the determination of cell viability by the addition of the MTT reagent according to the manufacturer's protocol (Roche, Basel, Switzerland; 11,465,007,001). Absorbance was measured at 550 nm. Viable cells were counted by trypan blue exclusion. Data were expressed as a percentage of the signal observed in vehicle-treated cells and are shown as the mean ± SEM of three independent experiments.
Irradiation
For in vitro experiments, 8505C cells were irradiated using a Faxitron X-ray (Faxitron Bioptics, AZ, USA) at 5 Gray (Gy), and treated with paclitaxel or lenvatinib alone, or the combination of paclitaxel and lenvatinib. For in vivo experiments, mice were treated using the Small Animal Radiation Research Platform (SARRP) [High-resolution, small animal radiation (RT) research platform with x-ray tomographic guidance capabilities/PMID; 18640502]. The tumors were irradiated with a circular beam of 1-cm diameter at 3 consecutive daily fractions of 3 Gy. (6 fractions of 0.5 Gy with 24-hour interval between each fraction) The current dose rate was 5.0 Gy/min at the center of the tumor.
Human Thyroid Cancer Cell Xenografts
KSA1 and 8505C cells (2.0x107 cells/mouse) were cultured in vitro and then injected subcutaneously into the upper left flank region of female BALB/c nude mice. After 15 days, tumor-bearing mice were grouped randomly (n=10/group) and treated with 25 mg/kg of paclitaxel (intraperitoneally) alone, 10 mg/kg lenvatinib (orally) alone, or a combination of 6.5 mg/kg paclitaxel and 5 mg/kg lenvatinib, for a total of 13 administrations. Tumor size was measured every other day using calipers. Tumor volume was estimated using the following formula: L2×S2 where, L represents the longest diameter and S refers to the shortest diameter). Animals were maintained under specific pathogen-free (SPF) conditions. All experiments were approved by the Animal Experiment Committee of Yonsei University.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Immunohistochemistry results were evaluated by ANOVA followed by Bonferroni post-hoc tests. Values are expressed as the mean ± SEM. P values < 0.05 were considered to indicate statistical significance.
Results
Inhibition of ATC by paclitaxel, lenvatinib, and radiation combination therapy
To examine the anti-cancer effects of paclitaxel, lenvatinib, and radiation therapy in combination, as well as those of paclitaxel or lenvatinib alone with radiation therapy on ATC, we assayed 8505C and KSA1 cell proliferation and viability in the presence or absence of these compounds using the MTT assay. Treatment with the combination of paclitaxel, lenvatinib, and radiation therapy had a lower IC50 in thyroid cancer than treatment with paclitaxel or lenvatinib and radiation therapy (Table 1, Fig. 1). Thus, these results suggest that the combination therapy offers a new approach for the treatment of ATC using low-doses of anti-cancer drugs.
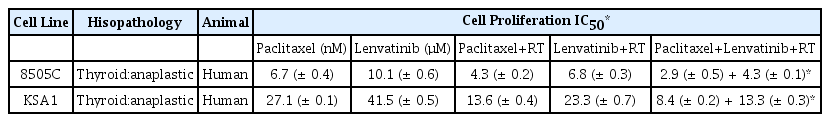
IC50 estimation with a cell proliferation assay treated Paclitaxel, Lenvatinib and RT-combined, Paclitaxel or Lenvatinib with RT on 8505C, KSA1. Each data point signifies the mean of 3 independent MTT assays for IC50 performed in triplicate. SD, standard deviation.
Combination of Paclitaxel, Lenvatinib and RT was suppressed cancer cell proliferation more efficiently than Paclitaxel or Lenvatinib with RT. Cell proliferation and viability assay of Paclitaxel, Lenvatinib and RT combined and Paclitaxel or Lenvatinib with RT in thyroid cancer (8505C, KSA1) cells. Points indicate mean % of the value observed in the solvent-treated control. All experiments were repeated at least 3 times. The data represent the mean ± SD. Experiments were repeated at least 3 times with similar results. * P < 0.05 vs. control, ** P < 0.01 vs. control, *** P < 0.005 vs. control.
Paclitaxel, lenvatinib, and radiation therapy combination induced caspase-mediated apoptosis of ATC
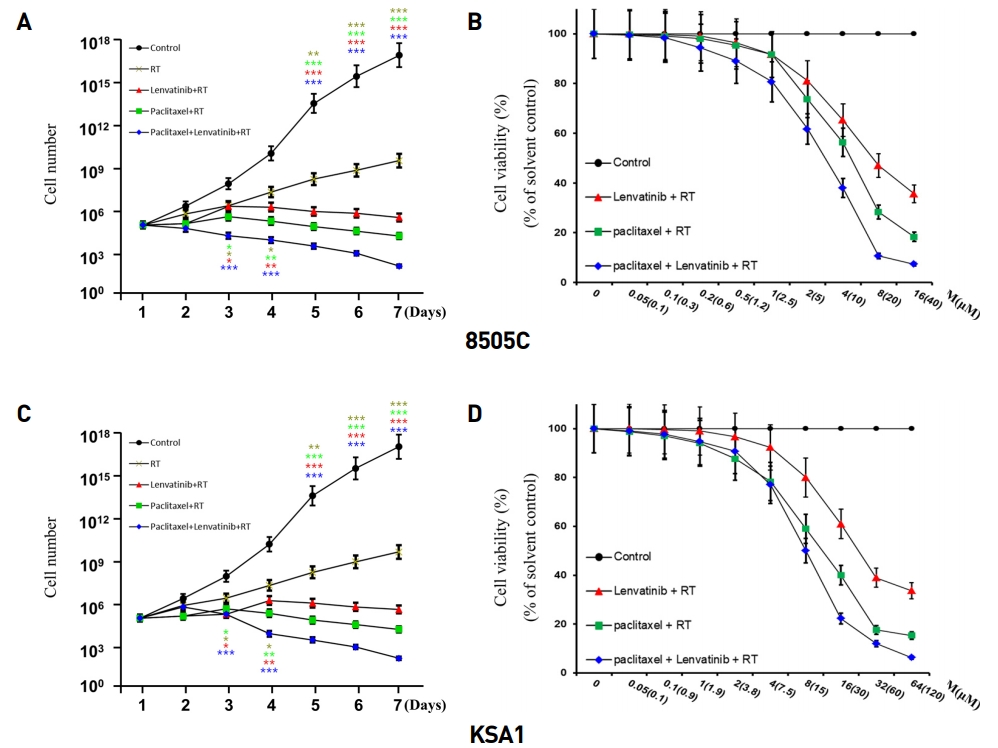
To evaluate the effect of the combination treatment consisting of paclitaxel, lenvatinib, and radiation therapy on apoptotic signaling pathways, we investigated the expression of the apoptotic proteins, Apaf-1 and cleaved-caspase 3, after treatment with these drugs by immunoblot analysis (Fig. 2). The combination treatment led to a significant increase in the levels of Apaf-1 and cleaved-caspase 3. Collectively, these data demonstrated that this drug combination efficiently induces apoptosis on ATC cell lines via caspase cleavage.
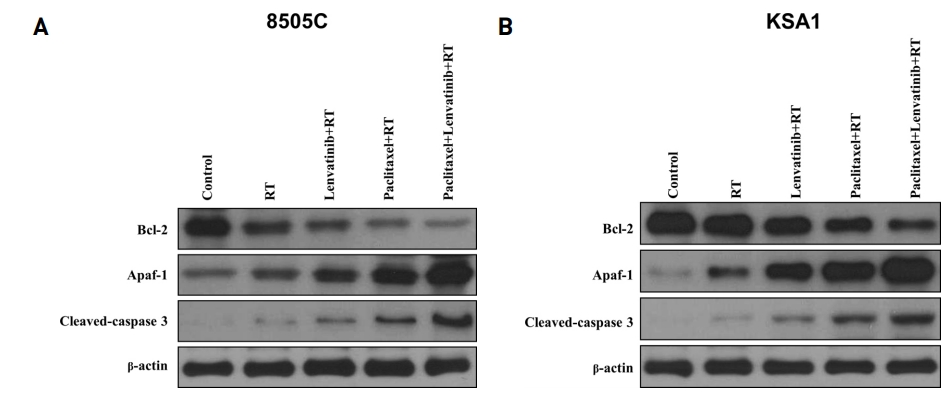
Combination of Paclitaxel, Lenvatinib and RT significantly induced cell cycle arrest and apoptosis. Immunoblot analyses suggested that the indicated Paclitaxel, Lenvatinib and RT increased the levels of apoptotic proteins and reduced those of anti-apoptotic proteins in thyroid cancer (8505C) cells. The Paclitaxel, Lenvatinib and RT combination potently induced the expression of cell cycle arrest proteins and reduced the expression of anti-apoptotic protein. KSA1 and 8505C were exposed to the indicated Paclitaxel, Lenvatinib and RT to the analysis of the expression of Apaf-1, cleaved-caspase 3 (apoptotic protein) by immunoblot analysis
Paclitaxel, lenvatinib, and radiation therapy combination suppressed tumor growth in a mouse xenograft model
Paclitaxel, lenvatinib, and radiation therapy combination markedly suppressed 8505C and KSA1 cell xenograft tumors. No evidence of systemic toxicity or treatment-related death was found in any group. There was no significant effect on the body weight of mice treated with paclitaxel, lenvatinib, and radiation therapy. The combination-treated group showed significantly smaller tumor volumes compared to the groups treated with paclitaxel or lenvatinib with radiation therapy.
This result demonstrated that the combination therapy caused a more efficient induction of apoptosis in mouse xenografts (Fig. 3). In conclusion, the combined effect of paclitaxel, lenvatinib, and radiation therapy is a new clinical approach for the treatment of ATC using low-doses of these anti-cancer drugs.
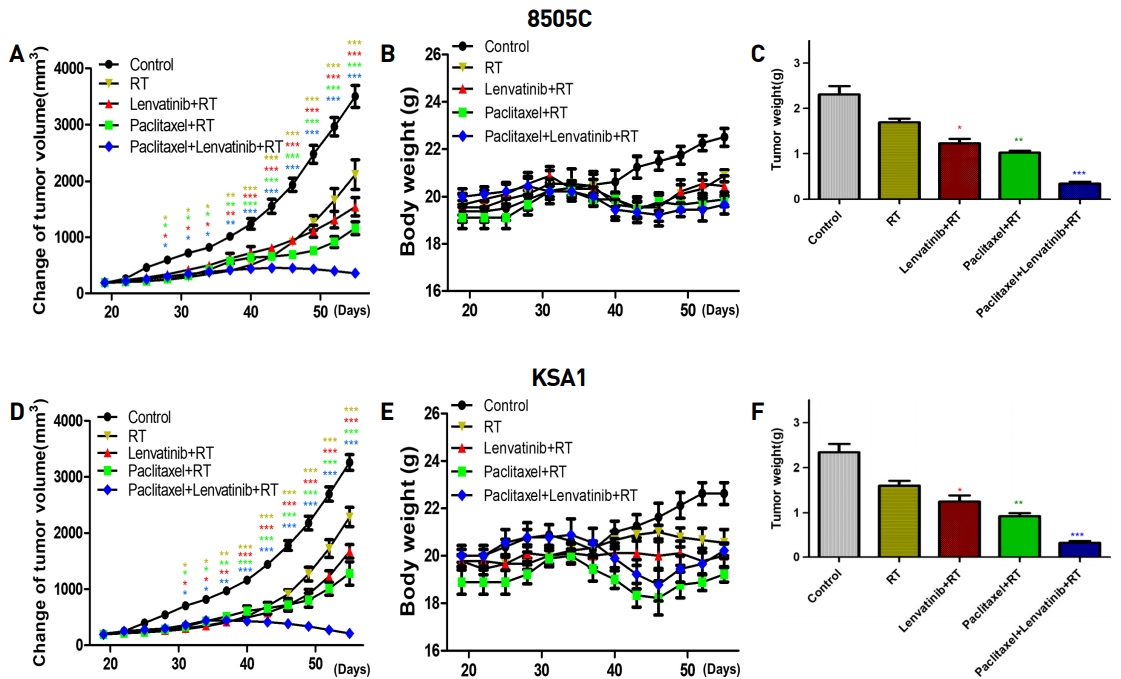
Combination of Paclitaxel, Lenvatinib and RT produced compelling anti-cancer effects in cancer cell xenografts in vivo. A thymic nude mice with established tumors were treated with the indicated drug or RT. Data represent the mean tumor volumes. Paclitaxel, Lenvatinib and RT combination therapy induced more potent inhibition of tumor progression than did Paclitaxel or Lenvatinib with RT in mice with thyroid cancer (8505C, KSA1) xenografts (n = 10 mice/group). No evidence of systemic toxicity or treatment-related death was found in Paclitaxel, Lenvatinib and RT combination-treated or Paclitaxel or Lenvatinib with RT groups. The compounds had no significant effect on mouse body weight (B). Weights of the dissected tumors (C). * P < 0.05 vs. control, ** P < 0.01 vs. control, *** P < 0.005 vs. control.
Discussion
ATC is an extremely rare form of undifferentiated cancer with a very high mortality rate [1,3]. Only complete surgical resection combined with adjuvant therapy such as radiation and chemotherapy is the main treatment option for ATC [8]. However, these therapeutic options have not been very effective and mortality still remains very high [4].
Many studies have examined the effect of the combination of several chemotherapeutic agents and tyrosine kinase inhibitors. A recent study has shown that lenvatinib, a tyrosine kinase inhibitor, potentiates the effect of paclitaxel against ATC in vitro and in vivo. The combined therapy synergistically inhibited colony formation and tumor growth and induced G2/M phase cell cycle arrest and apoptosis [22]. The combination of sorafenib and paclitaxel has also been tested in experimental models of breast cancer with bone metastases, resulting in anti-angiogenic, anti-tumor, and anti-resorptive effects [23]. Furthermore, TKI has been shown to sensitize hepatocellular carcinoma cells to sublethal doses of Taxol by knocking down hepatoma upregulated protein (HURP), which correlates with Taxol resistance [24].
Paclitaxel is known to have a radiosensitizing effect in various cancers. In adenocarcinoma cell lines, the mechanism underlying induction of radiosensitization may involve inhibition of DNA repair, cell cycle redistribution, and apoptosis [25,26]. The level of apoptosis after paclitaxel treatment may be considered a predictor of paclitaxel-induced radiosensitization [27]. In non-small cell lung cancer, adding low-dose paclitaxel as a radiosensitizer to radiation therapy improved outcomes compared to chemotherapy and radiation therapy alone [28]. Using paclitaxel as a cytotoxic drug and radiosensitizer may be useful in avoiding radiotoxicity by reducing the radiation dose.
Currently, many clinical trials are underway to test lenvatinib. A multicenter, randomized, double-blind, placebo-controlled, phase 3 trial of lenvatinib (E7080) in 131I-Refractory differentiated thyroid cancer showed improvement in progression-free survival in the lenvatinib group compared to the placebo group [29]. Recent studies have also reported the doses and interruption periods. The lenvatinib group with shorter dose interruption offered greater benefit than that with longer dose interruptions. Timely management of lenvatinib toxicities to minimize dose interruptions has been shown to be important [30].
The combination of paclitaxel, lenvatinib, and radiation therapy significantly increased Apaf-1 and cleaved-caspase 3. Collectively, these data demonstrated that the combination treatment was efficient in inducing apoptosis in ATC cell lines and that it exerts this effect through caspase cleavage. In addition, our results demonstrated that the paclitaxel, lenvanib and radiation therapy combination is the most efficient inducer of apoptosis in mouse xenografts. The mechanisms underlying these anticancer effects of the combination of these drugs require further investigation.
This study showed cytotoxic effects of the combination of paclitaxel, lenvatinib, and radiation therapy on ATC cell lines, both in vitro and in vivo. The combination therapy induced a more marked rise in the apoptosis of ATC cells than either lenvatinib or paclitaxel alone with radiation therapy. Consequently, these results indicate a novel approach for the treatment of cancers through a combination of low-dose anti-cancer drugs, which may help in reducing toxic side effects.
Acknowledgements
This work was supported by a faculty research grant from Yonsei University College of Medicine for 2018-31-0470, which was funded by Eisai Korea Inc.